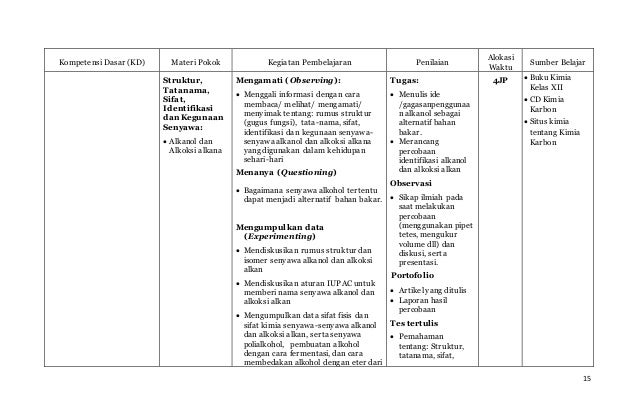
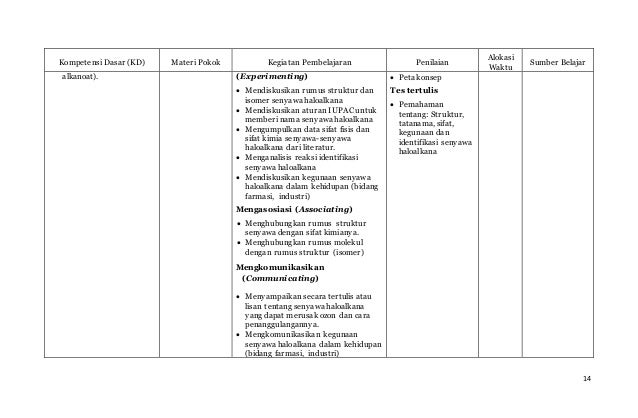
Mencari informasi dari literatur dan media farmasi tentang kegunaan senyawa karbon secara individu. MATA PELAJARAN. DEPARTEMEN PENDIDIKAN NASIONAL. DIREKTORAT JENDERAL MANAJEMEN PENDIDIKAN DASAR DAN MENENGAH. DIREKTORAT PEMBINAAN SMA. 2006 31 SILABUS KIMIA. Di samping faktor formulasi, cara pemberian obat turut menentukan cepat lambatnya dan lengkap tidaknya resorpsi obat oleh tubuh. Tergantung dari efek yang diinginkan, yaitu efek sistemis (di seluruh tubuh) atau efek lokal (setempat), keadaan pasien dan sifat – sifat fisika-kimia obat.
Kimia Analitik 2 Kimia Analitik 2 PENGANTAR SPEKTROSKOPI Sonny Widiarto Jurusan Kimia FMIPA Universitas Lampung Bahan Pelajaran / Silabus Kolorimetri dan Spektroskopi Radiasi elektomagnetik Sifat cahaya (sebagai gelombang - partikel) Absorpsi dan emisi Aspek kuantitatif (hukum Beer) Instrumen Spektrofotometri UV-Vis, IR dan serapan atom (AAS) Pustaka / referensi Harvey ch. 24 dan 25 (26-28) Pecsok ch. 8, 9, 10 (11-13) Kolorimetri dan Spektroskopi Radiasi Elektomagnetik Radiasi elektomagnetik merupakan kombinasi medan listrik dan medan magnet yang berosilasi dan merambat lewat ruang dan membawa energi dari satu tempat ke tempat yang lain Dualisme Gelombang-Partikel Cahaya adalah energi berbentuk gelombang elekromagnetik yang kasat mata dengan panjang gelombang sekitar 380–750 nm. Pada bidang fisika, cahaya adalah radiasi elektromagnetik, baik dengan panjang gelombang kasat mata maupun yang tidak. Selain itu, cahaya adalah paket partikel yang disebut foton. Kedua definisi tersebut merupakan sifat yang ditunjukkan cahaya secara bersamaan sehingga disebut 'dualisme gelombang-partikel' Electromagnetic radiation moves in waves Sifat gelombang 8 Light (called electromagnetic radiation) moves in waves.
Wavelength = different types of light have different wavelengths. Some are longer than others. For instance, in the visible light spectrum, red light waves are longer than blue light waves. Sifat partikel Interaksi Radiasi dan Materi Spektroskopi Spektrum Elektromagnetik Spektrum Elektromagnetik 17 Wavelengths are commonly given in????
Lambda λ Warna dan Panjang gelombang COLOR WAVELENGTH (λ in nm) Ultraviolet 780 Visible Light Colorimetry? The solutions of many compounds have characteristic colors. The intensity of such a color is proportional to the concentration of the compound. Spectroscopy and Spectrophotometry??
Light can either be transmitted or absorbed by dissolved substances Presence & concentration of dissolved substances is analyzed by passing light through the sample Spectroscopes measure electromagnetic emission Spectrophotometers measure electromagnetic absorption 21 The presence and concentration of various substances dissolved in a water sample is commonly analyzed by passing different types of light (visible, infrared, or UV) through the sample. Light can either be transmitted or absorbed by the dissolved substances. 22 Jenis – jenis Analytical Spectroscopy Absorption Fluoresence and Phosphoresence Emission (atomic with flames, arcs, sparks, and palsmas) Chemilumenesence and Biolumenesence Reflection Absorpsi Cahaya White light All colors Polychromatic light 23 When white (polychromatic) light passes through a coloured solution some of the light is absorbed by the substances in the solution, and the rest passes through. Green solution absorbs light other then green Monochromatic light Light of one color Red light is absorbed by the green solution 24 If white light is made to pass through a red filter, all light except red is filtered out and absorbed. Therefore, only red light hits the solution.
The Spectrophotometer 25 The monochromatic light is obtained by allowing the beam of light to pass through a prism or diffraction grating (monochrometer) The monochromatic light is directed through a cuvette containing the sample, and the light that penetrates hits the photoelectric cell. The current developed by the photoelectric cell is translated into percent transmission or absorbance through a galvanometer. Then you can read the absorbance on the galvanometer. Absorbance is also called extinction and optical density In review, a spectrophotometer is used to measure the absorption of a certain color of light (specific wavelength) as it passes through a treated sample. A compound of interest is reacted with reagents creating a colored substance. It is the blockage or absorption of light by this secondary material (dye) that is actually measured.

 0 kommentar(er)
0 kommentar(er)
